How to get DMEPOS Accreditation
This DMEPOS Accreditation guide is for informational purposes only. SuretyBonds.com does not regulate or manage licensing for DMEPOS accreditation. Contact the Centers for Medicare & Medicaid Services for the latest official DMEPOS accreditation requirements.
Medical suppliers who distribute durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) must be accredited with the Centers for Medicare & Medicaid Services (CMS) to verify they meet required program quality standards. All DMEPOS suppliers must be accredited and comply with DMEPOS quality standards to keep their Medicare billing privileges.
Complete the following steps file for your Centers for Medicare & Medicaid Services DMEPOS Accreditation.

How do I get DMEPOS Accreditation?
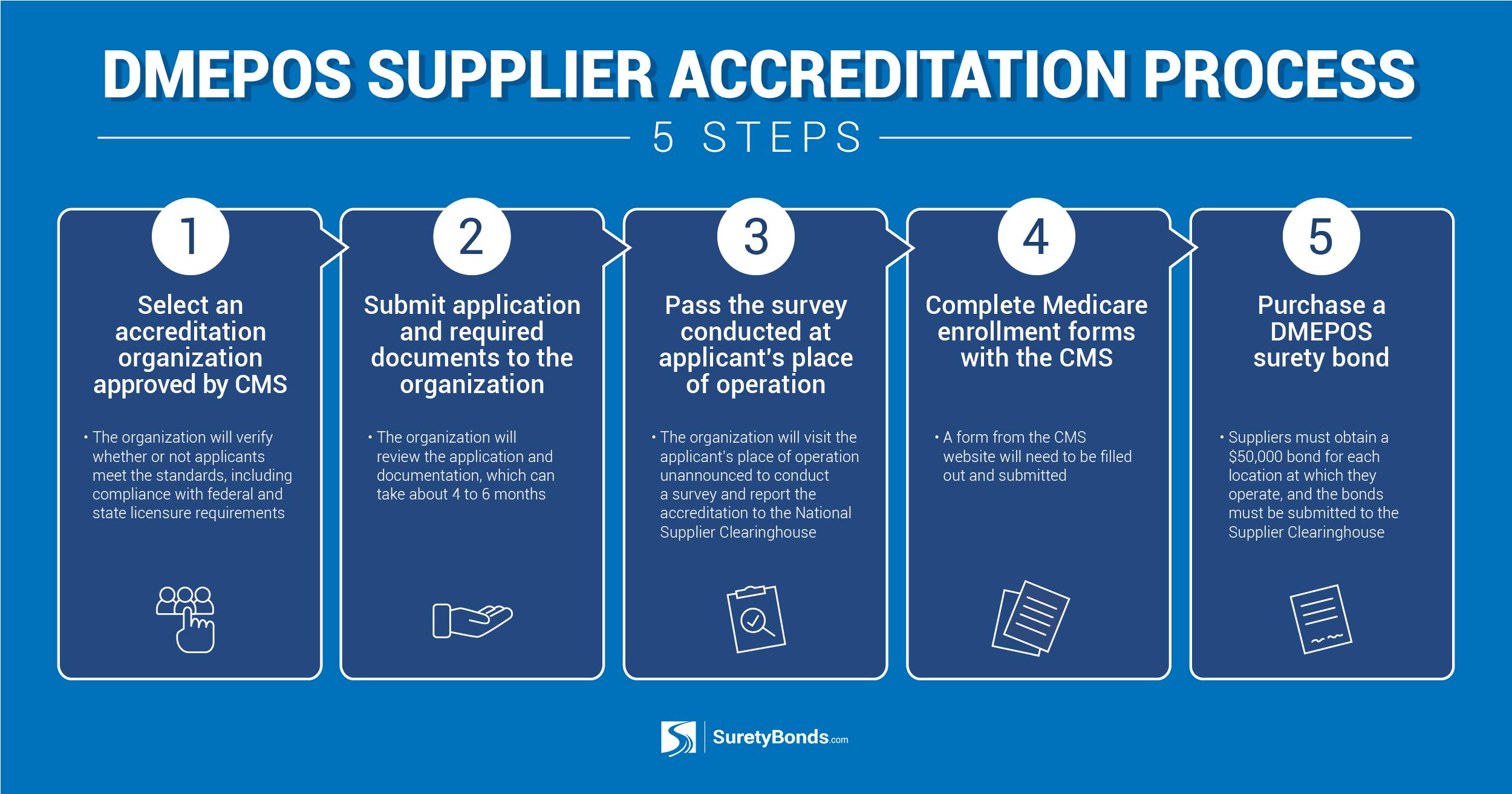
Step 1: Select an accreditation organization approved by CMS.
You must select an CMS-approved accreditation organization to guide you through the accreditation process. These organizations verify whether you meet the required DMEPOS quality standards and help you determine what changes to make to ensure compliance. For example, DMEPOS suppliers must provide complete and accurate information on their DMEPOS supplier application and comply with federal and state licensing requirements. You can also review this CMS-approved DMEPOS Accreditation Organizations list.
Step 2: Submit your application and required supporting documentation to your accreditation organization.
Once any necessary changes have been made to your application, you must submit it to your accreditation organization along with all required supporting documentation. Your accreditation organization will review your submission and verify all credentials meet CMS standards, which can take 4 to 6 months.
Step 3: Pass your business location survey.
Once your application and supporting documents have been reviewed, your accreditation organization will visit your business location unannounced to conduct a survey. A surveyor from the National Association of Boards of Pharmacy will visit to determine whether you meet all required standards. If your business location meets all requirements, the accreditation organization will report your DMEPOS supplier accreditation to the National Supplier Clearinghouse, which is the organization that issues and revokes billing privileges for DMEPOS suppliers. If your business location does not meet all required standards or falsified information is found on your application, your DMEPOS supplier accreditation will be denied.
NOTE: Accreditation organizations conduct unannounced onsite surveys at least every 3 years.
Step 4: Complete your Medicare enrollment forms with CMS.
After your business location survey has been processed by the National Supplier Clearinghouse, you'll apply for your Medicare enrollment with the Centers for Medicare & Medicaid Services. You'll need to complete the correct enrollment form from the CMS website depending on the service you offer as a DMEPOS supplier.
Step 5: Purchase your DMEPOS surety bond.
Suppliers must purchase and file a $50,000 DMEPOS surety bond for each location they operate. DMEPOS bonds protect consumers from being taken advantage of by suppliers and guarantee the legitimacy of claims submitted to Medicaid, thus cracking down on fraud on the part of the supplier. The bond also guarantees money is available to any parties harmed by the supplier’s failure to adhere to all rules and regulations.
You can apply for your DMEPOS surety bond online 24/7, with annual premiums starting at $250. Once your order has been processed, your official DMEPOS surety bond will be emailed to you.
What do I do after I get my DMEPOS surety bond?
You must keep all enrollment information current with CMS to prevent your Medicare billing privileges from being revoked. You must report any change within 30 days, including the following.
- business location
- ownership
- adverse legal action
If you applied for your CMS DMEPOS accreditation online, you can update your information directly in the Medicare Provider Enrollment, Chain, and Ownership System (PECOS). If you applied using the paper application, you'll need to submit physical paperwork to update your information.
What is Medicare?
The Medicare program provides federal health insurance for individuals 65 and older and some individuals under 65 who have certain conditions or disabilities.
What is Medicaid?
The Medicaid program provides health coverage for some individuals who have limited incomes or other resources.
Last Updated: March 7, 2024
Have Questions?
Call 1 (800) 308-4358 to talk with a Surety Expert today.
